ACCELERATE Paediatric Strategy Forums: An Advance for Oncology Drug Development?
A new article published by the ACCELEATE Platform highlights how prioritisation of new anti-cancer drugs to accelerate drug development in children and adolescents with cancer can be achieved by multi-stakeholder discussion. ACCELERATE has organized nine Paediatric Strategy Forums since 2017, initially with the collaboration of the European Medicines Agency (EMA) and subsequently with the participation of the U.S. Food and Drug Administration (FDA). The Forums have highlighted the optimal development of pathway in diseases/medicines where prioritisation is needed through international cooperative academic groups working in partnership with advocates and pharmaceutical companies.
“In a landscape of mechanism of action-driven drug development, the large number of medicinal products available for adults and the limited size of the relevant population of children mandates prioritisation of products, with the acceleration of the development of some classes of products and waiving others”, states the paper published in The Lancet Oncology.
Have the last nine Forums achieved their goal?
After reviewing the rationale and conclusions of the Forums, the paper concludes that the last nine editions have successfully achieved their goals. “The Forums have resulted in prioritisation and conclusions both specific to the topic of the Forum and more generally about criteria relevant to prioritisation and helped to frame future discussions between industry and regulators”, state the authors.
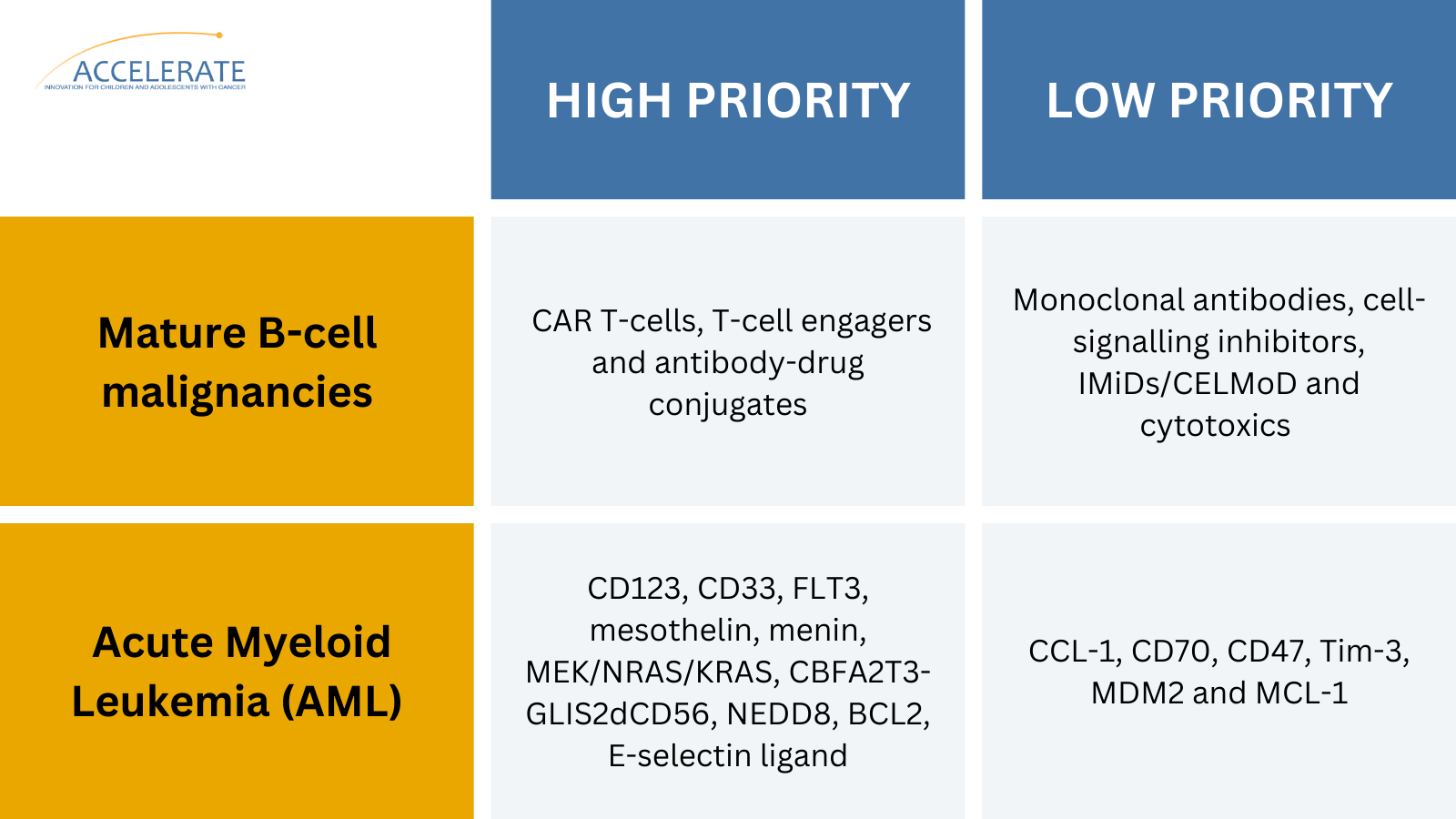
Two examples of outcomes from the ACCELERATE Paediatric Strategy Forums. Read all the conclusions here.
In addition, the Forum on epigenetic modifiers concluded that development of menin inhibitors should be accelerated in children with cancer. Two industry-supported, academic-sponsored global platform trials have also been launched as a result of the Forums
What are the ACCELERATE Paediatric Strategy Forums?
The ACCELERATE Paediatric Strategy Forums share information and facilitate dialogue between all stakeholders to inform paediatric drug development strategies and subsequent integration of the clinical perspective into the development efforts. “This prioritisation, as with all aspects of the development of new anti-cancer drugs in children, demands an integrated approach of all stakeholders - academia, industry, regulatory agencies, and patient advocates as equal partners”, reads the paper. The concept of Paediatric Strategy Forums has the potential to be of value beyond paediatric oncology, as highlighted by the EMA and PDCO.
Who participates in the Forums? International academic experts present the landscape, regulators actively participate but no regulatory advice is provided nor regulatory decisions made, patient advocates are key contributors, and pharmaceutical companies present available data. In total there have been 1,019 participants and 134 products have been discussed.
The paper strongly encourages simultaneous regulatory submissions and inter-regulatory discussion at cluster calls between Europe and the US. “In Europe, involvement of health technology assessment bodies early in development will be crucial to facilitate patient access to newly approved drugs. Where there are multiple similar products of the same class, a focused and sequential approach has been proposed”, conclude the authors. To discover more, read the full publication here.







