The Critical Role of Academic Clinical Trials in Pediatric Cancer Drug Approvals
A new study published by the ACCELERATE Fit For Filing Working Group reports that academic sponsors and industry partners need to prospectively recognize when the planned collaborative trial could contribute to an application to marketing authorization and plan accordingly. “Transparent collaboration and knowledge sharing between the partners opens an important pathway for accelerating new treatments into clinical practice for children with cancer”, states the paper published in the Journal of Clinical Oncology.
How can these collaborative studies contribute to marketing authorizations? What are the obstacles and possible solutions to enable academic-sponsored trials to directly contribute to the licensing of new medicines?
In their latest publication, the FFF Working Group has identified critical gaps in knowledge, standard procedures, and resources that hindered the trial data directly contributing to marketing authorization applications.
When are early phase or new trials appropriate or needed for FFF?
The consideration of FFF should be taken at the time of trial development (not after the trial has been initiated or completed) and should be based on:
- The goals of the trial
- What other studies are underway or planned
- The patient population and how they were selected
- The proposed end points
- Many other variables
What are the knowledge and expertise gaps?
A filing application requires levels of validation and traceability of not just the study data but of the entire process from data collection, data oversight (including query management), risk management, and document management.
The surveys conducted by the FFF Working Group revealed differences between academia and industry in the approach to essential data, critical for the evaluation of the safety, toxicity, and efficacy profile of a drug or medicinal product. It also highlighted differences in the data collection processes, data cleaning, and review strategies.
What are the recommendations?
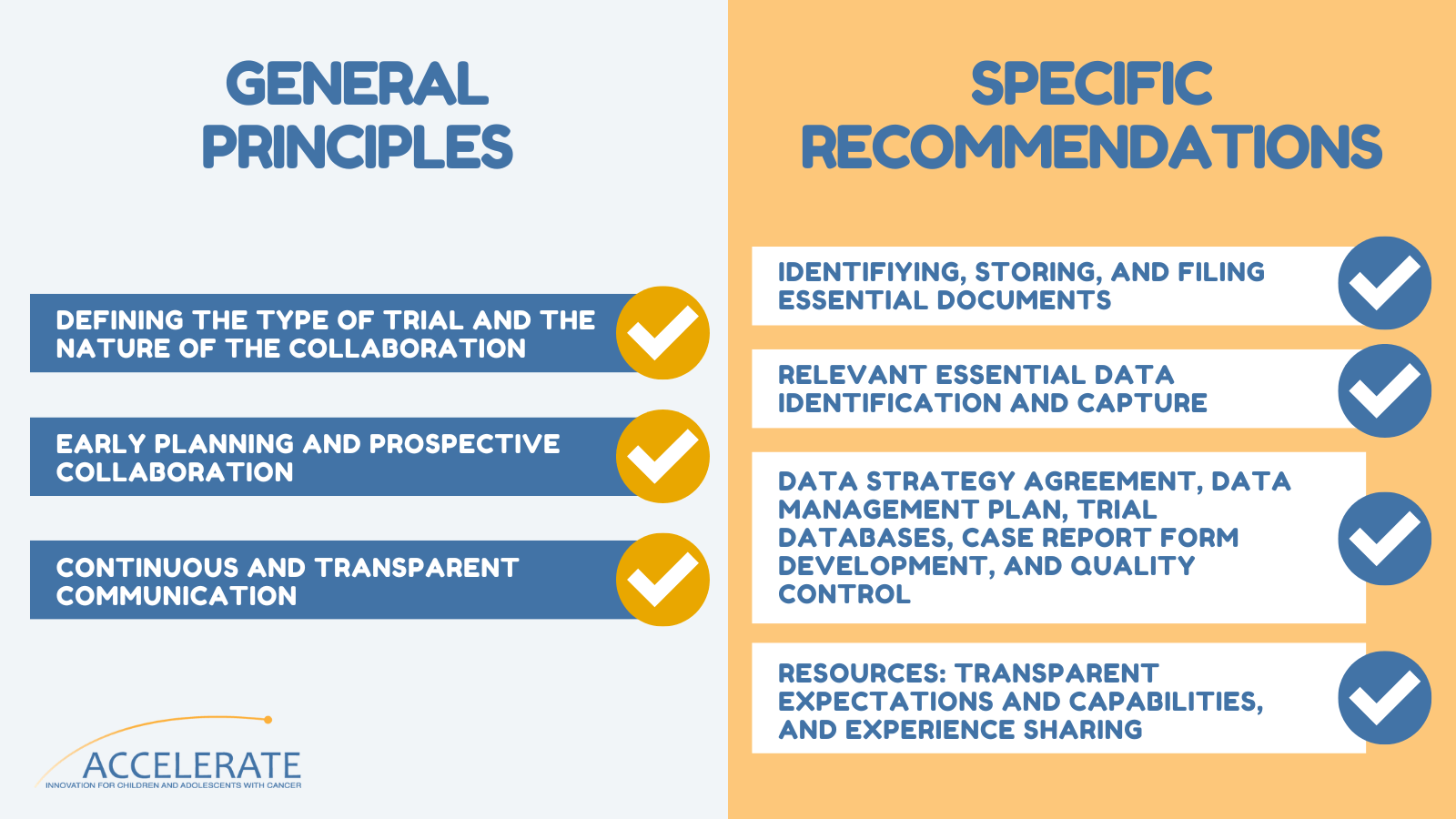
The study concludes that the goal of clinical drug development in pediatric oncology is to provide ready access to new drugs that will improve the likelihood of survival and decrease treatment-emergent side effects. “Successful collaboration between industry and academic investigators with early input from regulatory agencies is necessary, and the inclusion of advocate engagement to ensure patient-centric focus is encouraged”. To dive into the critical role of academic clinical trials in pediatric cancer drug approvals, read the full publication here.







