International collaboration
About
International collaboration in clinical trials is essential to develop new and better drugs for children and adolescents with cancer. During the first part of 2021, the International Collaboration (ICO) working group has worked tirelessly toward the achievement of its objectives.
The International Collaboration Working Group has been established by ensuring the involvement of the relevant target groups: it includes 12 Academia, 4 Pharma, 2 Regulators and 4 Patient Advocates.
Who we are
The International Collaboration WG is currently co-led by Dr Greg Reaman (FDA), Nicole Scobie (Zoe4Life) & Dr Teresa De Rojas (ACCELERATE).
The Working group brings together representatives of four stakeholder groups:
Academia (clinicians and researchers):
Steve Hunger (Children's Hospital of Philadelphia), Martin Schrappe (University Hospital Kiel), Andrea Biondi (Research Center for leukaemic and haematological diseases of children and of “S. Verri” Cellular and Gene Therapy Laboratory), Tom Gross (The Ohio State University), Alberto Pappo (St. Jude Children's Research Hospital), Maryam Fouladi (Brain Tumor Translational Research), Brenda Weigel (Children's Oncology Group (COG)), Jim Whitlock (Hospital for Sick Children), Kathy Brodeur Robb (C17 Council), Maria Grazia Valsecchi (University of Milano-Bicocca), Todd Alonzo (COG), Peter Wejbora (Childrens Cancer Institute), Geoffrey McCowage (Australasian Children's Cancer Trials)
Regulators:
Dominik Karres (EMA)
Industry (pharmaceutical companies and biotechs):
Darshan Wariabharaj (Janssen), Elly Barry (Day One Biopharmaceuticals), Anjali Sharma (Amgen), Kathleen Neville (Johnson & Johnson)
Advocacy (parents, patients and survivors):
Donna Ludwinski (Solving kids Cancer, Leona Knox (Solving Kids Cancer UK), Vicki Beugner (Coalition Against Childhood Cancer)
For more information, please contact the working group lead:
International Collaboration Working Group Secretariat: carole.lecinse@gustaveroussy.fr
Work packages
WP-1: Analysis of international clinical trials for children with cancer – completed.
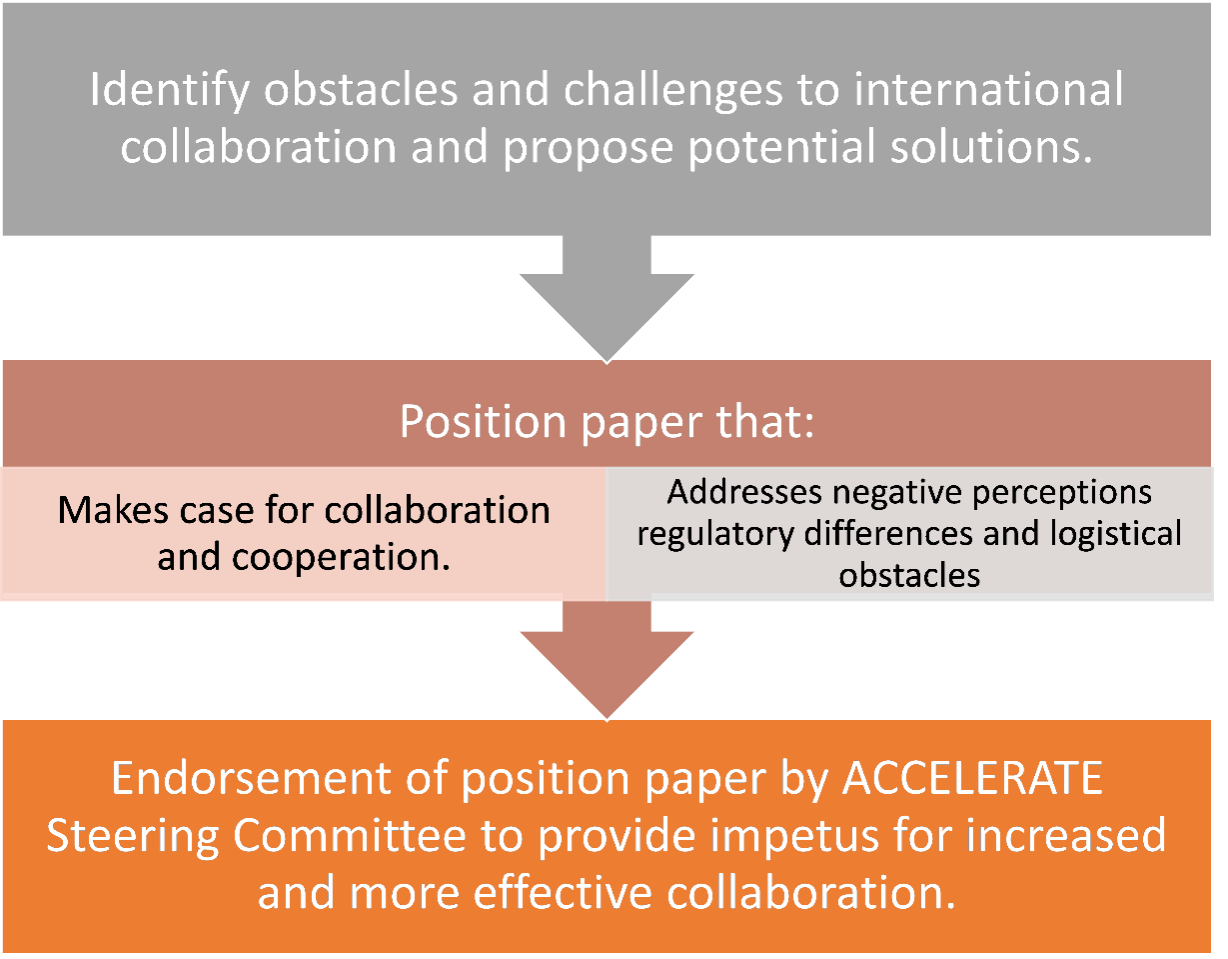
The results of this landscape analysis will be soon published in a scientific journal: There is an alarming lack of intercontinental collaboration which is essential to develop more and better drugs for kids with cancer.
WP-2: International survey with multi-stakeholder experts – ongoing.
With the international survey with multi-stakeholder experts, we aim to understand why there are so few intercontinental trials, identifying the challenges and hurdles.
WP-3: Multi-stakeholder discussion and consensus – ongoing.
The ICO working group worked intensively to align its work with existing international collaboration initiatives (such as within ITCC) and establishing collaborations with these organisations.
Next steps: Once the hurdles to international collaboration are identified (WP-2), this working group will analyze all gathered data and propose solutions to overcome those hurdles (WP-3).