FAIR TRIALS
Why FAIR trials
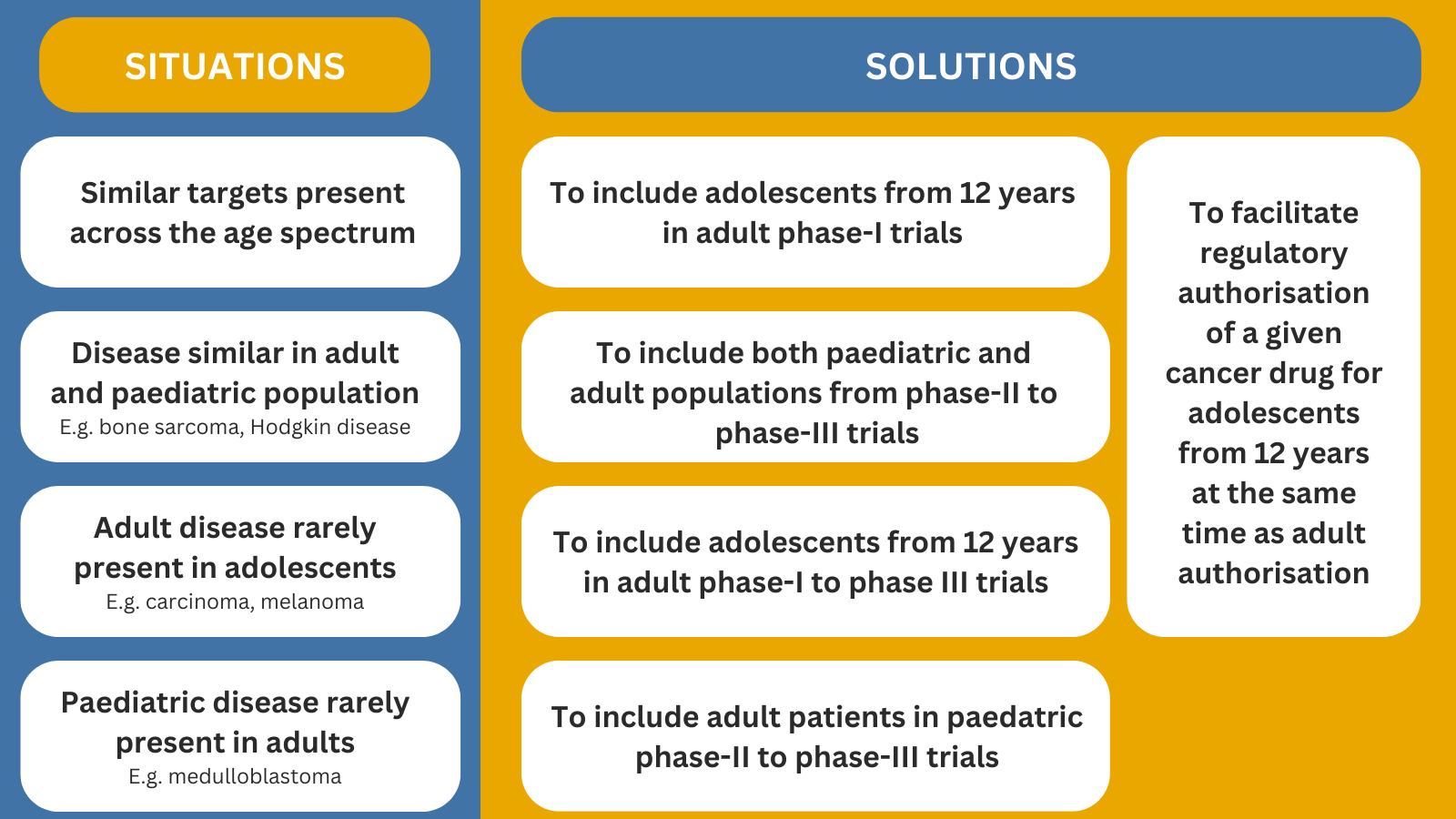
Research into teenage and young adults’ cancer has made slower progress than for any other age group. One reason is that many clinical trials exclude patients under 18 – without medical justification.
To confront this problem, the Working Group on Fostering Age Inclusive Research (or FAIR Trials) was established in 2017 by the ACCELERATE Forum. Made up of 15 experts from Academia, Industry and Parents/Patients advocacy, the group aims to raise awareness and promote change.
We propose a six-point approach in order to improve access for adolescents to new anti-cancer drugs and to make the drug development process more efficient. Read more about the six-point approach here.
Positions open!
Would you like to join the ACCELERATE FAIR Working Group? We are currently looking for:
- Medical Oncologists
- Patient and Parent Advocates
Whether you work in Pharma, Research, Ethics or as a Patient Advocate, join our circle of supporters so that we can share expertise and experience.
Push the arguments in your own networks. Patient advocates are often consulted on the planning of research trials and these are ideal opportunities to bring the issues to the table.

FAIR for AYA stamp

Four clinical trials in oncology have been awarded the FAIR for AYA STAMP, a new accolade that credits medical research which actively avoids unnecessary barriers based on age. It is hoped that the STAMP will encourage other researchers to offer opportunities for adolescents and young adults to participate in trials.
What is the objective?
The FAIR Trials initiative aims to accelerate innovation in drug development for young people with cancer, through the removal of arbitrary age limits in clinical trials.
E-learning
Take a look at the ESMO-SIOPE E-Learning tool Improving AYA Access To Innovative Therapies by Breaking the 18 Years Dogma.
Learning Objectives
- To raise awareness about needs for new drugs for haemato-oncology treatment in adolescents and young adults.
- To provide an overview of the European regulation and current landscape in the drug development and early drug access in the adolescent and young adult population.
- To provide an overview of proposed changes in trial strategy for adolescents and young adults, from early drug development to accelerate AYA access to the therapeutic innovation, by the FAIR (Fostering Age Inclusive Research) trial group of the multi-stakeholder European Paediatric Platform ACCELERATE.
Survey on early phase trials (phase I/II) for adolescents and young adults with cancer
Are you part of academia, industry or health authorities? Have you experienced potential hurdles associated with inclusive early phase trials in the field of new drug development? The FAIR Group survey is just designed for you!
Our goal is to encourage access of adolescent and young adults (AYA) into adult phase I/II innovative trials where appropriate based on the disease or mechanism of action of the drug but also access of young adults (>18) into paediatric early phase trials, especially when their diagnosis is a ‘paediatric’ type cancer.
A set of practical resources to assist in designing age inclusive clinical trials.
What can you find in the toolkit?
- FDA Draft Guidance for Industry
- eCRF and Standard Analyses
- Patient Reported Outcomes (PROs)
- Assent templates for adolescents
- Protocol Elements
- Examples of HA/EC considerations on AYA
- List of AYA-clinical sites
ADVOCACY TOOLKIT
Publications
FAIR Group publications
Joint adolescent–adult early phase clinical trials to improve access to new drugs for adolescents with cancer: proposals from the multi-stakeholder platform—ACCELERATE
Gaspar, N. et al. (2018)
Annals of Oncology
FAIR trials for Adolescents & Young Adults Scientific publications
Ease the Age Barrier That Keeps Young Cancer Patients Out of Trials. Applied Clinical Trials
O’Donnell, P., Feb.2020.
The 18-year-old clinical trial inclusion limit is a major barrier in the access to immunotherapies and targeted therapies for adolescents and young adults (AYAs) with cancer.
De Rojas, T., Neven, A., Garcia-Abos, M., Moreno, L., Gaspar, N., & Peron, J. 2019. Annals Of Oncology, 30 (Supplement_1)
FAIR trials for Adolescents & Young Adults in Mainstream media
Who we are
The FAIR WG is currently co-led by Nathalie Gaspar & Max Williamson.
The Working group brings together representatives of four stakeholder groups:
Academia (clinicians and researchers):
Martin Elliott, Leeds University
Nathalie Gaspar (co-Chair), Gustave Roussy
Lynley Marshall, Royal Marsden
Elizabeth Corley, Royal Marsden
Julia Glade Bender, MSKCC
Cristina Larrosa, Sant Joan de Deu
Aditi Vedi, Addenbrookes Cambridge (UK)
Claudia Valverde Morales, Vall d’Hebron University Hospital - Medical Oncologist
Viswatej Avutu, MSKCC - Medical Oncologist
Industry (pharmaceutical companies and biotechs):
Christina Buccirechtweg, Novartis
Solange Corriol-Rohou, AstraZeneca
Evgenia Mengou, EV Pharma Solutions Ltd.
Mark Kieran, Day One
Stergios Zacharoulis, BMS
Gabriele Bieska, Roche
Brian Friend, Astellas Pharma Inc.
Advocacy (parents, patients and survivors):
Max Williamson
Ana Amariutei
Deanna Fournier
Ann Graham
Julie Guillot
Menia Koukougianni, KARKINAKI (Greece)
For more information, please contact the working group lead:
FAIR Working Group Secretariat: Carole Lecinse carole.lecinse@gustaveroussy.fr
Chris Copland – Parent Representative
Clinical trials offer hope that one day we may save the thousands of young lives we lose to cancer every year in Europe alone. However, currently, many trials exclude patients under eighteen, though this age limit bears little relation to science, safety or compassion.
What are the consequences?
Delays. New treatments for conditions like lymphoma, only become available to adolescents years after they have been authorised for adults.
Nathalie Gaspar – Gustave Roussy
As a paediatric oncologist, my main fear is of missing an opportunity that might benefit my patients by giving them a chance of either cure or control their disease. A patient with cancer not being able to access a trial just because he/she is under 18 has become unbearable.
I have listened to adolescents telling me, “Why have you prescribed this drug to my mate of 19 years old and you are telling me that I cannot have access to this drug without travelling miles away?”